With ten approved influenza vaccines, on the U.S. market right now, across three main production methods — eggs, cell cultures, and recombinant protein, do we need a genetic mRNA product?
The annual influenza vaccine campaign led by the CDC has become a major public health failure with minimal efficacy and rising concerns of the shots causing more colds and flu while raising the risks of serious conditions like paralysis from Guillain-Barre Syndrome. This story was outlined by Alter AI.
.
.
.
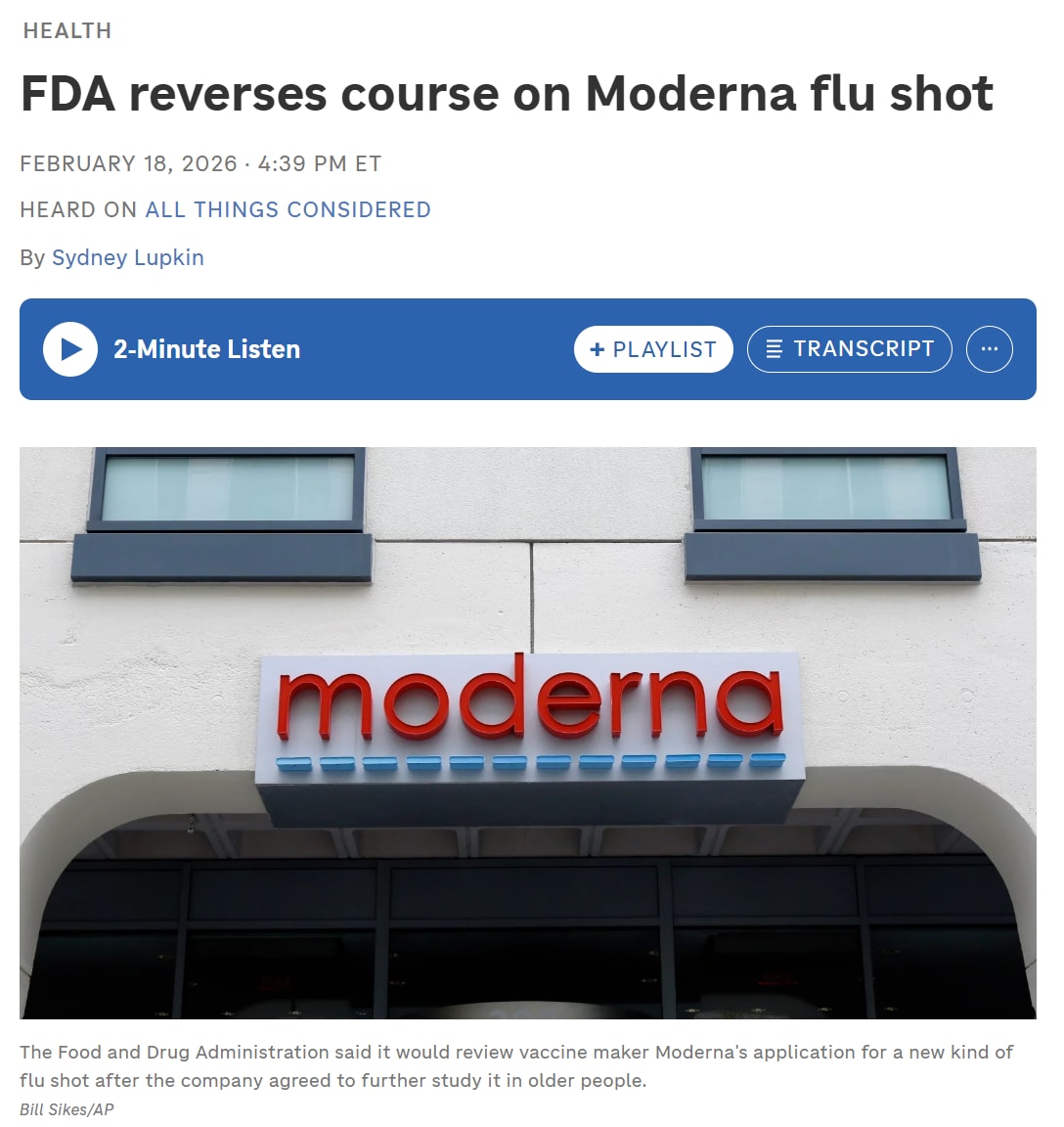
In a stunning about-face, the U.S. Food and Drug Administration (FDA) has reversed its earlier refusal to review Moderna’s new mRNA-based influenza vaccine, mRNA‑1010 — after the company and its lobbyists reportedly held high-level meetings with agency officials. The decision comes just a week after the FDA’s own top vaccine regulator, Dr. Vinay Prasad, rejected the original application, citing a flawed clinical design that used an outdated, low-dose flu vaccine as the control—not a placebo, violating the agency’s own standards for “adequate and well-controlled” trials. Prior to his current post, Prasad had no significant drug or vaccine regulatory experience and was known as a podcaster who mocked attempts at early treatment for COVID-19.
According to reports, the FDA’s reversal occurred after Moderna proposed splitting its application into two parts: full approval for adults aged 50‑64 and a conditional “accelerated” approval for those 65 and older. That change satisfied enough bureaucratic criteria to reopen the review, but critics within the agency remain wary. Insiders who supported the original rejection note that Moderna’s trial did not test its shot against a placebo, which means its safety and efficacy comparisons are questionable.
The broader context exposes an increasingly politicized battle over vaccines. Health and Human Services Secretary Robert F. Kennedy Jr. has pushed regulators to apply higher scrutiny to mRNA technologies, while major pharmaceutical firms and investors have argued that such caution “chills innovation.” Yet, despite these warnings, Moderna’s persistent lobbying appears to have paid off. Its stock immediately jumped over 6% following the FDA’s reversal — a reaction that underscores the financial stakes tied to regulatory leniency.
Many scientists, including career FDA staff, quietly question whether this decision represents genuine scientific reconsideration or industry muscle flexing. The agency’s reversal conveniently clears the way for Moderna’s future combination Covid-flu shot, a potential multi‑billion‑dollar product. Meanwhile, the lack of long-term data on mRNA platforms for seasonal vaccination raises lingering safety concerns — particularly among older adults, the very group targeted by Moderna’s initial filing.
This episode highlights a familiar pattern in U.S. drug regulation: corporate influence reshaping scientific judgment. As one FDA veteran privately put it, “If a smaller company had tried to submit that same data, they wouldn’t even get a meeting.”
The agency’s final decision on Moderna’s flu vaccine is now expected by August 5, 2026—but the question remains whether the review will reflect rigorous evaluation or capitulation to pharmaceutical power.
*
Click the share button below to email/forward this article. Follow us on Instagram and X and subscribe to our Telegram Channel. Feel free to repost Global Research articles with proper attribution.
Global Research is a reader-funded media. We do not accept any funding from corporations or governments. Help us stay afloat. Click the image below to make a one-time or recurring donation.